On patch testing and the T.R.U.E. test
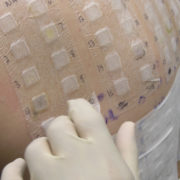
What is Patch Testing? Patch testing is a method for diagnosing delayed type hypersensitivity. Chemicals (haptens) are applied on the skin with wells adhered to an adhesive strip. The wells may be hand-loaded (‘comprehensive’) patch testing or may be preloaded in gels (‘patch test kit’, e.g.: T.R.U.E. test). They are then left in contact with the skin for up to 48h, then removed and checked. They are then re-evaluated between 72-120h (sometimes later). Some reactions can appear later and the patient should monitor their back for new reactions for up to 8 weeks. If a new reaction presents, they should notify their provider.
What is the T.R.U.E. test? The thin-layer rapid epicutaneous (T.R.U.E.) testT. is a commercially available patch test kit that is approved for use in adults in the US by the Food and Drug Administration.
What is it used for? It is used to confirm the diagnosis of allergic contact dermatitis (ACD) – a delayed hypersensitivity reaction.
How effective is the test? Data from the North American Contact Dermatitis Group (NACDG), a US-based research group of patch testers, suggest ” Using a conservative calculation (assuming that individual components of a mix would not have been detected with a mix and vice versa), the conservative hypothetical detection rate of T.R.U.E. TEST allergens would be 69.7%”. Which means that theoretically ~70% of the reactions that the NACDG detected with their screening panel would have been also captured if the T.R.U.E. test were used. We say ‘theoretically’ because they are different systems and can have inherent differences in detection rates (see below). One limitation of patch testing (in general) is that it is a confirmatory test, so you can only detect what is tested for. Testing the patients products (shin-guards, personal hygiene products, medicaments) is an important adjunct to the standard patch testing, and can provide vital information about additional potential allergens and their sources. http://www.ncbi.nlm.nih.gov/pubmed/25581671
How does patch system compare to comprehensive testing (methods)? There are variations within the patch test systems:
Comprehensive testing – Comparative study of IQ-ultra and Finn Chambers: “Both patch tests had a significant agreement in detecting all of the allergens. An “almost perfect agreement” was noted for ethylenediamine dihydrochloride, quaternium-15, mercapto mix, black rubber mix, balsam of Peru, and nickel sulfate; “substantial agreement” for formaldehyde, bisphenol A epoxy resin, and 4-tert-butylphenol formaldehyde resin; and “moderate agreement” for potassium dichromate.” : http://www.ncbi.nlm.nih.gov/pubmed/22417991
T.R.U.E. test versus Comprehensive testing; “Discordant positive reactions were examined for clinical relevance. The Finn Chamber methodology was superior in detecting clinically relevant allergies to fragrance mix, balsam of Peru, and thiuram mix. T.R.U.E. Test performed somewhat better than the Finn Chamber in detecting relevant allergic reactions to nickel, neomycin, and methylchloroisothiazolinone/methylisothiazolinone. Neither T.R.U.E. Test nor Finn Chamber methodologies performed optimally in detecting relevant allergies to formaldehyde and carbamates. Practitioners limited to only the T.R.U.E. Test methodology need to be aware that relevant reactions to fragrances, rubber accelerators/pesticides (carbamates and thiurams), and formaldehyde may be missed with this system.” http://www.ncbi.nlm.nih.gov/pubmed/11712026
Methyisothiazolinone may be missed: “The clinical manifestations of allergy to MCI/MI and MI are highly variable and diagnosis is often missed. In the standard patch test series of the Spanish Contact Dermatitis and Skin Allergy Research Group (GEIDAC), MCI/MI is tested at 100 ppm, but at this concentration, up to 50% of cases might go undetected. Furthermore, our data indicate that MCI/MI at 200 ppm would make it possible to diagnose more cases of contact allergy to MI. To improve the diagnosis of contact allergy to MCI/MI and MI, we believe that the test concentration of MCI/MI should be increased to 200 ppm in the GEIDAC standard series and that MI should be added in the GEIDAC standard series.” http://www.ncbi.nlm.nih.gov/pubmed/24626102
Additional information: http://www.ncbi.nlm.nih.gov/pubmed/24117737
Fragrances can be volatile and should be loaded into the patch chamber as close in time to the application to the patient as possible “Within a couple of hours several fragrance allergens evaporate from test chambers to an extent that may affect the outcome of the patch test. Application to the test chambers should be performed as close to the patch test occasion as possible and storage in a refrigerator is recommended.” : http://www.ncbi.nlm.nih.gov/pubmed/22803625
For more information on contact dermatitis and patch testing – please visit the Dermatitis Academy at https://www.dermatitisacademy.com